Mechanical control of chemical reactions
Molecular level control of chemical reactions (as opposed to macroscopic thermodynamic control) is a requisite for networking many reactions together in the same volume, as in the cell. Our Lab pioneered the mechanical control of enzymes as a universal, modular control mechanism for chemical reactions.
Enzymes are molecular catalysts: a region of the surface of the enzyme (usually a "pocket") provides the catalytic environment for a specific reaction. Enzymes are also deformable molecules: the molecular structure can exist in essentially a continuum of different conformations. These are accessible by applying mechanical stress to the molecule. The last two statements adumbrate a general molecular mechanism to modulate enzymatic reactions, by applying mechanical stresses which deform the enzyme. There are approximately 104 known different enzymatic reactions; each one can in principle be thus subjected to molecular control!
A practical realization of these ideas is the method of the DNA spring, described below. We synthesize an enzyme − DNA chimera, a supra-molecular construction where a single-stranded DNA oligomer (typically 40 – 60 bases long) is covalently attached by the ends to two specific surface sites on the enzyme (labeled by Cys residues introduced by mutagenesis). Hybridization to the complementary strand rigidifies the DNA spring, which exerts a mechanical stress on the enzyme. Thus we can modulate the enzymatic activity.
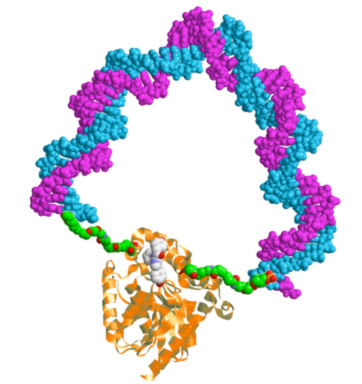
At left: Cartoon of a protein-DNA chimera; the protein (Renilla Luciferase) and DNA spring are drawn approximately to scale.
The first important result to come out of these experiments is the realization that the enzyme may be regarded as continuously deformable.
Our measurements with the DNA spring show that different parameters of the enzymatic reaction are affected differently by different applied stresses: so there must be many different states accessible through the mechanical stress [Tseng 2010].
One consequence, of interest to experimentalists, is that at the simplest level it does not matter where one attaches the molecular spring on the enzyme: there will typically be some modulation of activity.
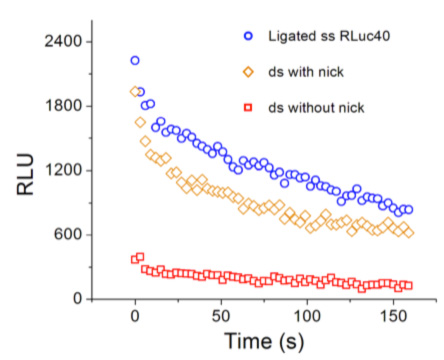
At right, speed of the enzymatic reaction catalyzed by the enzyme Renilla Luciferase (the chimera of Fig. 1) under different states of mechanical stress imparted by the DNA spring. This chemical reaction is easily quantified because a visible photon is emitted. Plotted is the light intensity emitted in the course of time (the speed of the reaction is time dependent because the concentrations of substrate and product evolve in time). Circles: no stress; diamonds: small stress; squares: larger stress.
From control to measurement: the chemo – dynamic cycle of enzymes. Substrate binding to an enzyme also results in a mechanical stress which deforms the enzyme: this is Koshland’s mechanism of induced fit. Binding – unbinding of substrates and products thus drives a cycle of deformations of the enzyme. This cyclic conformational motion is the basis for enzymes to operate as molecular machines. The DNA spring allows to probe the conformational cycle, since it couples to molecular deformations. One step in this program is the study of how mechanical stress affects the forward and reverse reactions catalyzed by the same enzyme. A first report appeared here [Joseph 2014]. Ultimately the goal is to establish universal properties of the chemo – dynamic cycle of enzymes. Some ideas are proposed here [Qu 2013].
Associated publications:
- B. Choi, G. Zocchi, Y. Wu, S. Chan, L. Jeanne Perry, “Allosteric control through mechanical tension”, Phys. Rev. Lett. 95, 078102 (2005).
- G. Zocchi, “Controlling proteins through molecular springs”, Ann. Rev. Biophys. 38, 75-88 (2009).
- C-Y. Tseng, A. Wang, and G. Zocchi, “Mechano-chemistry of the enzyme Guanylate Kinase”, Europhys. Lett. 91, 18005 (2010).
- Hao Qu and Giovanni Zocchi, “The complete bending energy function for nicked DNA”, Europhys. Lett. 94, 18003 (2011).
- H. Qu and G. Zocchi, “How Enzymes Work: A Look through the Perspective of Molecular Viscoelastic Properties”, Phys. Rev. X 3, 011009 (2013).
- C-Y. Tseng and G. Zocchi, “Mechanical control of Renilla Luciferase”, J. Am. Chem. Soc. 135, 11879 (2013).
- C-Y. Tseng and G. Zocchi, “Equilibrium softening of an enzyme explored with the DNA spring”, Appl. Phys. Lett. 104, 153702 (2014).
- C. Joseph, C-Y. Tseng, G. Zocchi and T. Tlusty, “Asymmetric effect of mechanical stress on the forward and reverse reaction catalyzed by an enzyme”, PLoS ONE 9, e101442 (2014).